Recombinant Human Beta-crystallin S (CRYGS
-
中文名称:Recombinant Human Beta-crystallin S(CRYGS)
-
货号:CSB-EP006025HU
-
规格:¥1344
-
图片:
-
其他:
产品详情
-
纯度:Greater than 90% as determined by SDS-PAGE.
-
基因名:CRYGS
-
Uniprot No.:
-
别名:AI327013; Beta-crystallin S; CRBS_HUMAN; CRYG8; crygs; Crystallin; gamma 8; Crystallin; gamma polypeptide 8; Crystallin; gamma S; CTRCT20; Gamma crystallin S; Gamma S crystallin; Gamma-crystallin S; Gamma-S-crystallin; Opacity due to poor secondary fiber cell junction; recessive nuclear cataract; Opj; rncat
-
种属:Homo sapiens (Human)
-
蛋白长度:Full Length of Mature Protein
-
来源:E.coli
-
分子量:47.9kDa
-
表达区域:2-178aa
-
氨基酸序列SKTGTKITFYEDKNFQGRRYDCDCDCADFHTYLSRCNSIKVEGGTWAVYERPNFAGYMYILPQGEYPEYQRWMGLNDRLSSCRAVHLPSGGQYKIQIFEKGDFSGQMYETTEDCPSIMEQFHMREIHSCKVLEGVWIFYELPNYRGRQYLLDKKEYRKPIDWGAASPAVQSFRRIVE
Note: The complete sequence including tag sequence, target protein sequence and linker sequence could be provided upon request. -
蛋白标签:N-terminal GST-tagged
-
产品提供形式:Liquid or Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
缓冲液:If the delivery form is liquid, the default storage buffer is Tris/PBS-based buffer, 5%-50% glycerol.
Note: If you have any special requirement for the glycerol content, please remark when you place the order.
If the delivery form is lyophilized powder, the buffer before lyophilization is Tris/PBS-based buffer, 6% Trehalose. -
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet & COA:Please contact us to get it.
相关产品
靶点详情
-
功能:Crystallins are the dominant structural components of the vertebrate eye lens.
-
基因功能参考文献:
- aberrant modifications in gammaS-crystallin structure might contribute to the lower stability and higher aggregatory potency of the mutated protein, which subsequently resulted in cataracts in the patients PMID: 29857103
- The Tyr67Asn substitution was predicted to decrease the local hydrophobicity and affect the three-dimensional structure of gammaS-crystallin, and resulted in a portion of mutant protein translocation from the cytoplasm to cell membrane. This observations expand the mutation spectrum of CRYGS and provide further evidence for the genetic basis and molecular mechanism of congenital cataract. PMID: 29964096
- Cataract-related G18V point mutation affects CRYGS stability and hydration. PMID: 27052457
- novel mutation (G57W) in CRYGS in this Chinese family is associated with autosomal dominant pulverulent cataract. PMID: 24328668
- The data suggest that enhanced attractive protein-protein interactions, arising from the deamidation of HGS, promote protein aggregation, thereby leading to increased light scattering and opacity over time. PMID: 26158710
- The effects of the V41M mutation on the structural changes of gamma S-crystallin were studied. PMID: 24287181
- The cataract-associated mutant D26G of human gammaS-crystallin is remarkably close to the wild type molecule in structural features, with only a microenvironmental change in the packing around the mutation site. PMID: 23761725
- replacement of valine in position 42 by the longer and bulkier methionine in human gammaS-crystallin perturbs the compact beta-sheet core packing topology in the N-terminal domain of the molecule PMID: 23284690
- age-dependent cleavage of gammaS-crystallin generates a peptide that binds to cell membranes PMID: 22995907
- The degree of deamidation for Gln92 and Gln170 was found to increase from birth to teen-age years and then to remain constant for four decades. PMID: 22593035
- Molecular dynamics (MD) simulations, circular dichroism (CD), and dynamic light scattering (DLS) measurements were used to investigate the aggregation propensity of the eye-lens protein gammaS-crystallin. PMID: 21244846
- Partially folded aggregation intermediates of human gammaD-, gammaC-, and gammaS-crystallin are recognized and bound by human alphaB-crystallin. PMID: 20621668
- Deamidation in cataractous lenses is influenced by surface exposure. PMID: 12093281
- A lens gamma S-crystallin has been identified with an in vivo modification, S-methylation of cysteine residues, that may block intermolecular disulfide bondng and serve as a form of protection against cataract. PMID: 12475213
- when glutathione becomes bound to gammaS-crystallin, it causes it to bind in turn to the beta-crystallin polypeptides to form a dimer PMID: 14763903
- report a novel missense mutation, p.V42M, in CRYGS associated with bilateral congenital cataract in a family of Indian origin PMID: 19262743
- Fast charge transfer quenching is an evolved property of the gamma S-crystallin fold, probably protecting it from ultraviolet-induced photodamage. PMID: 19358562
- Results confirm the high stability of wild-type HgammaS-crystallin and demonstrates that the G18V mutation destabilizes the protein toward heat and GuHCl-induced unfolding. PMID: 19558189
显示更多
收起更多
-
相关疾病:Cataract 20, multiple types (CTRCT20)
-
蛋白家族:Beta/gamma-crystallin family
-
数据库链接:
Most popular with customers
-
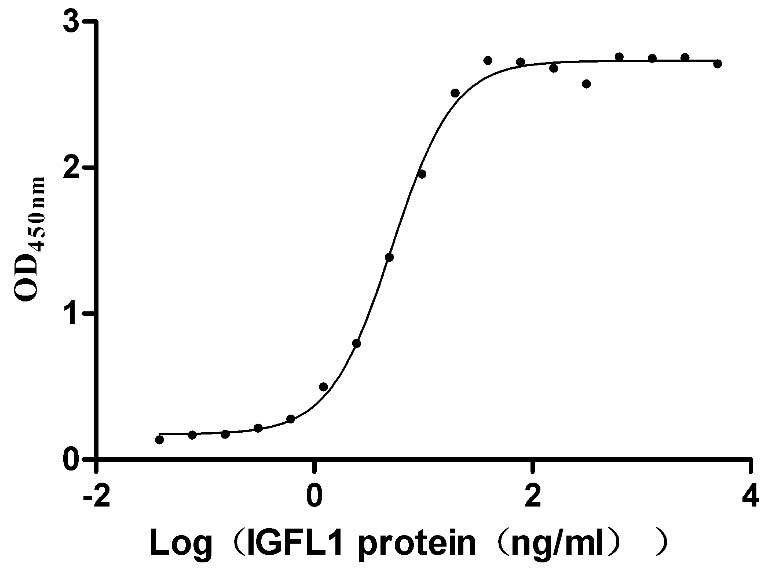
Recombinant Human IGF-like family receptor 1 (IGFLR1), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
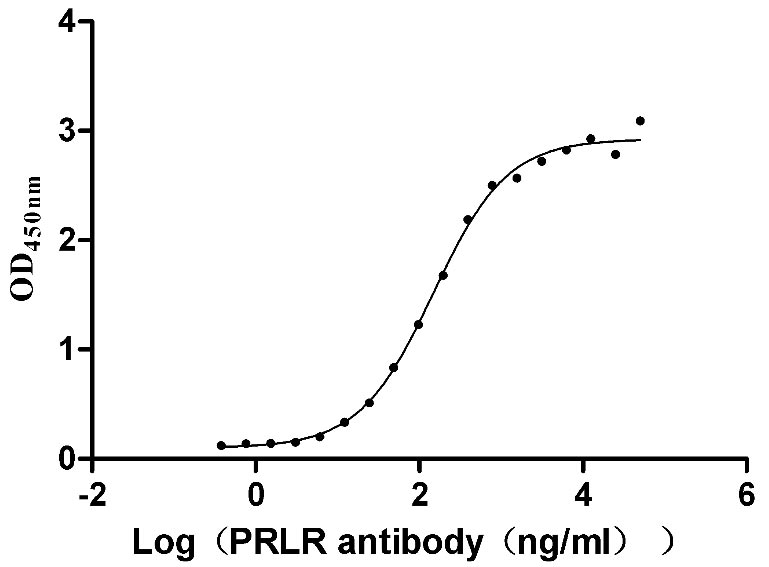
Recombinant Human Prolactin receptor (PRLR), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Melanoma-associated antigen 4 (MAGEA4) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Claudin-6 (CLDN6)-VLPs, Fluorescent (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
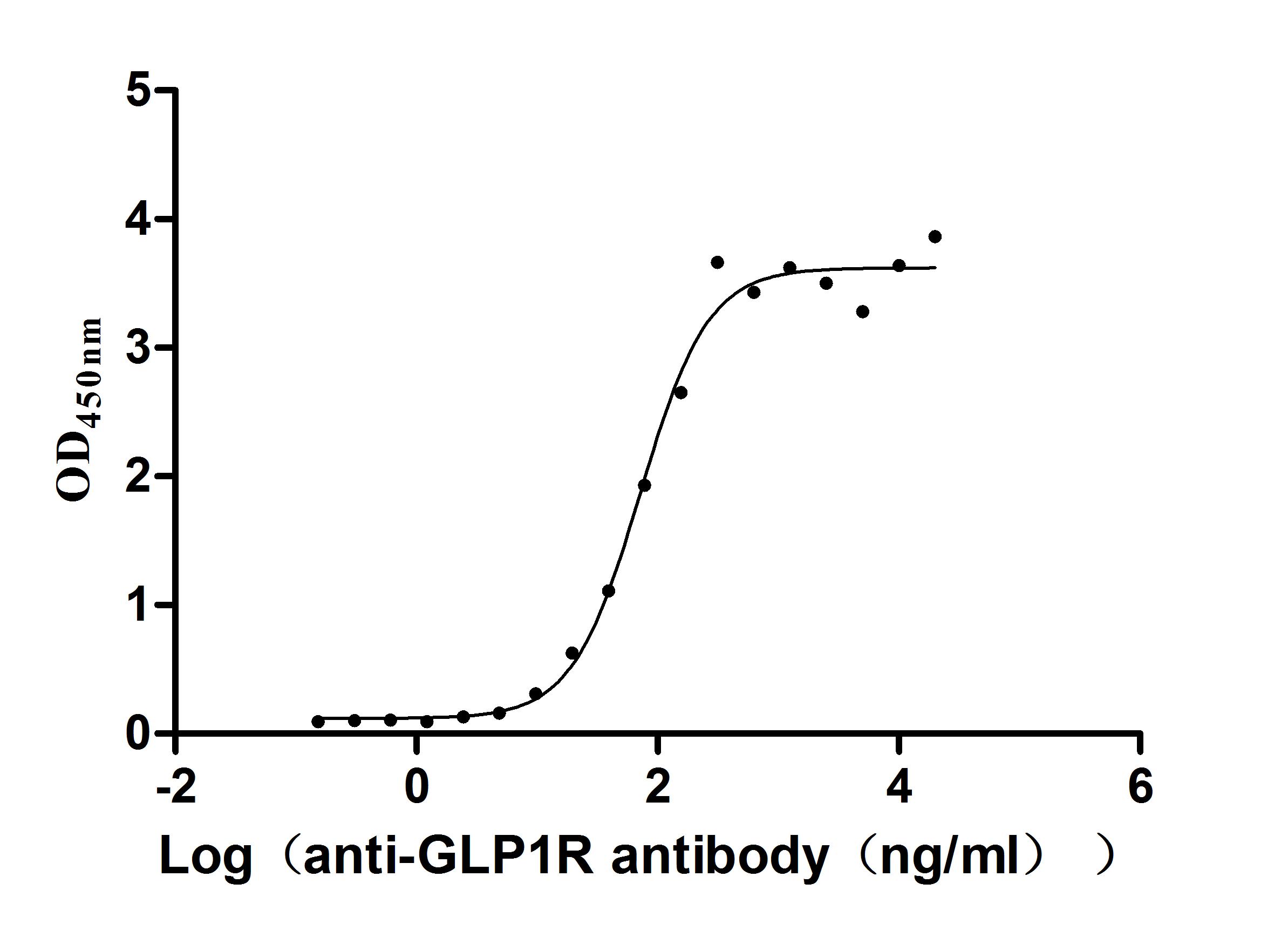
Recombinant Human Glucagon-like peptide 1 receptor (GLP1R), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
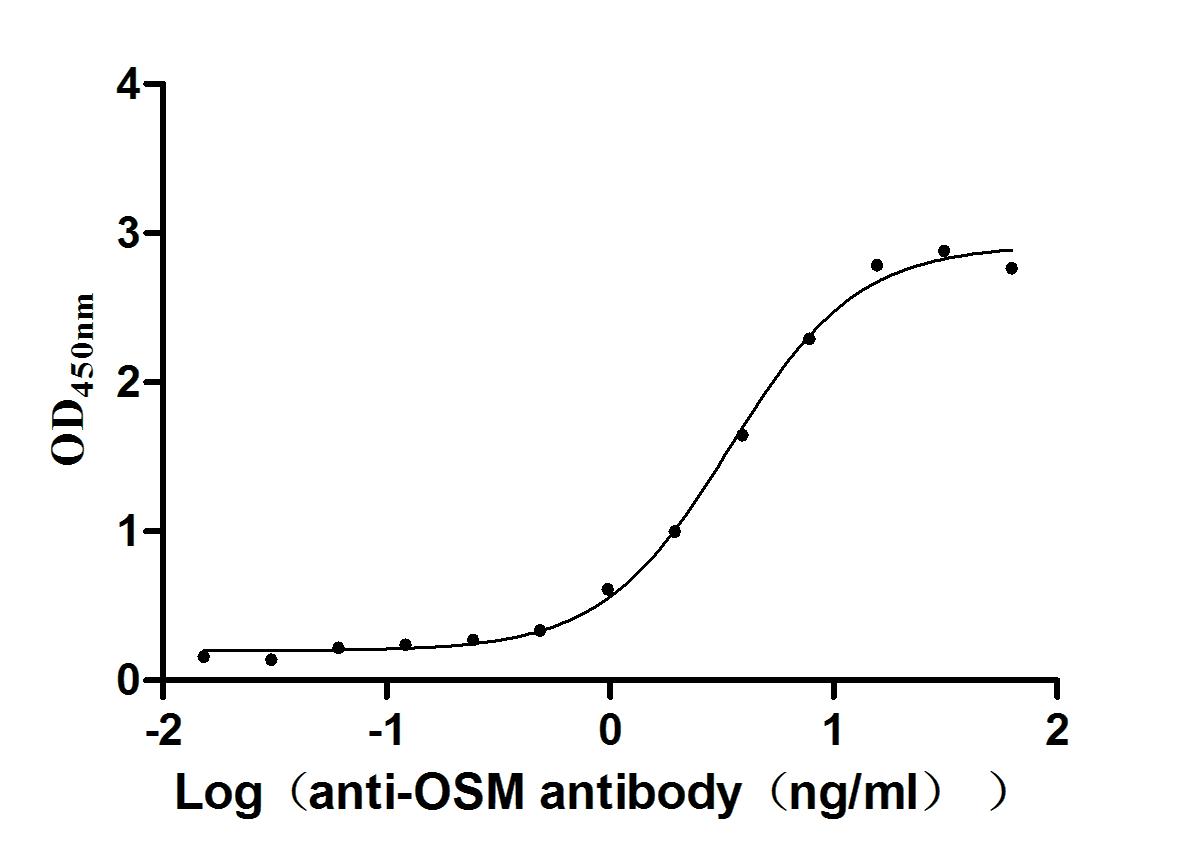
Recombinant Human Oncostatin-M (OSM), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Cytotoxic and regulatory T-cell molecule (CRTAM), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)



f4-AC1.jpg)