Recombinant Saccharomyces cerevisiae ATP-dependent molecular chaperone HSP82 (HSP82), partial
-
中文名称:酿酒酵母HSP82重组蛋白
-
货号:CSB-YP365784SVG
-
规格:
-
来源:Yeast
-
其他:
-
中文名称:酿酒酵母HSP82重组蛋白
-
货号:CSB-EP365784SVG
-
规格:
-
来源:E.coli
-
其他:
-
中文名称:酿酒酵母HSP82重组蛋白
-
货号:CSB-EP365784SVG-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名称:酿酒酵母HSP82重组蛋白
-
货号:CSB-BP365784SVG
-
规格:
-
来源:Baculovirus
-
其他:
-
中文名称:酿酒酵母HSP82重组蛋白
-
货号:CSB-MP365784SVG
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:HSP82
-
Uniprot No.:
-
别名:HSP82; HSP90; YPL240C; ATP-dependent molecular chaperone HSP82; 82 kDa heat shock protein; Heat shock protein Hsp90 heat-inducible isoform
-
种属:Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Baker's yeast)
-
蛋白长度:Partial
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
靶点详情
-
功能:Molecular chaperone that promotes the maturation, structural maintenance and proper regulation of specific target proteins involved in cell cycle control and signal transduction. Undergoes a functional cycle that is linked to its ATPase activity. The nucleotide-free form of the dimer is found in an open conformation in which the N-termini are not dimerized and the complex is ready for client protein binding. Binding of ATP induces large conformational changes, resulting in the formation of a ring-like closed structure in which the N-terminal domains associate intramolecularly with the middle domain and also dimerize with each other, stimulating their intrinsic ATPase activity and acting as a clamp on the substrate. Finally, ATP hydrolysis results in the release of the substrate. This cycle probably induces conformational changes in the client proteins, thereby causing their activation. Interacts dynamically with various co-chaperones that modulate its substrate recognition, ATPase cycle and chaperone function. Required for growth at high temperatures.
-
基因功能参考文献:
- Folding and domain interactions of three orthologs of Hsp90 proteins have been reported. PMID: 29276035
- In this study, the authors have established that Chl1, the protein which is involved in maintaining sister chromatid cohesion as well as in preventing chromosome loss, is a direct client of Hsp90. PMID: 29875144
- Results define two sites on Hsp82: the N-terminal domain (SdN) and C-terminal domain (SdC) which seem to be important for different aspects of the Hsp90 reaction cycle that are regulated by Sti1. SdN function seems to promote a physical interaction of Hsp90 with substrate-bound Hsp70 and SdC-region functions by establishing an Hsp90 conformation crucial for capturing clients and progressing through the reaction cycle. PMID: 29930177
- Of 98 derived amino acid states that evolved along this lineage, about half compromise fitness when introduced into the reconstructed ancestral Hsp90. And the vast majority of ancestral states reduce fitness when introduced into the extant S. cerevisiae Hsp90. PMID: 29626131
- results revealed a major role of Nt-acetylation in the Hsp90-mediated protein homeostasis, a strong up-regulation of the Arg/N-end rule pathway in the absence of NatA, and showed that a number of Hsp90 clients are previously unknown substrates of the Arg/N-end rule pathway PMID: 28515311
- Using crosslinking, hydrogen exchange mass spectrometry, and fluorescence experiments, we demonstrate here that the N-terminal domain of Hsp90 rotates by approximately 180 degrees as compared to the crystal structure of yeast Hsp90 in complex with Sba1 and AMPPNP. PMID: 28363677
- This study used Hsp90 mutants that modulate ATPase activity and biological function as probes to address the importance of conformational cycling for Hsp90 activity. The study found no correlation between the speed of ATP turnover and the in vivo activity of Hsp90: some mutants with almost normal ATPase activity were lethal, and some mutants with lower or undetectable ATPase activity were viable. PMID: 27723736
- The impact of different allosteric modulators on the stability, structural and internal dynamics properties of Hsp90 closed state was used to develop a quantitative model relating Hsp90 activation to the presence of a certain compound, using information on the dynamic adaptation of protein conformations to the presence of the ligand, which allows the capture of conformational states relevant in the activation process. PMID: 27032695
- that, similar to the Hsp70 family, the Hsp90 chaperones also influence yeast prion maintenance, and that immunophilins could regulate protein multimerization independently of their activity as peptidyl-prolyl isomerases PMID: 26473735
- HSP90 binding regulates Sti1 conformation. PMID: 25851214
- deletion of a charged linker region in HSP82 causes a pronounced defect Rad51-dependent DNA repair. PMID: 25380755
- High-resolution NMR solution structures of Tah1 free and in complex with the Hsp90. PMID: 24012479
- Binding of the glucocorticoid receptor modulates the conformational cycle of Hsp90. PMID: 24613341
- we systematically investigated the fitness effects of point mutations in a putative substrate binding loop of yeast Hsp90 (Hsp82) over a broad range of expression strengths. PMID: 23825969
- Suggest that Hsp90 provides an evolutionarily conserved mechanism that links environmental stress to the induction of yeast morphological diversity. PMID: 23434373
- Charge rich domain in Hsp82p promotes cell growth and maturation of model clients (substrates). PMID: 22660624
- A specific mutation in yeast Hsp90, hsc82-W296A, resulted in altered transcription patterns genetically linked to the cAMP pathway. PMID: 22461145
- adaptive value of Hsp90's effects on the relationship between genotype and phenotype PMID: 21205668
- Hsp90 and the cochaperone Sba1/p23 accumulate in the nucleus of quiescent Saccharomyces cerevisiae cells. PMID: 19889838
- ATP binding and hydrolysis by Hsp90 are both required for the efficient maturation of glucocorticoid receptor and v-Src. PMID: 19906642
- effect of Hsp90 mutations on binding and ATPase regulation by the co-chaperones Aha1, Sti1 (Hop), and Sba1 PMID: 15466438
- An extended network, consisting of 198 putative physical interactions and 451 putative genetic and chemical-genetic interactions, was found to connect Hsp90 to cofactors and substrates involved in a wide range of cellular functions PMID: 15766533
- potentiates the acquisition of fluconazole resistance; required to maintain resistance acquired by rapid selection but not resistance acquired by gradual selection PMID: 16195452
- analysis of intrinsic inhibition of the Hsp90 ATPase activity PMID: 16461354
- Data show that under normal growth conditions, Hsp90 plays a major role in various aspects of the secretory pathway and cellular transport; during environmental stress, Hsp90 is required for the cell cycle, meiosis, and cytokinesis. PMID: 17923092
- Hsp82p promotes telomerase DNA association and facilitates DNA extension once telomerase is engaged with the DNA PMID: 17954556
- The conformational transitions are orders of magnitude slower than the ATP-hydrolysis step and thus are the limiting events during the reaction cycle. PMID: 19234467
- Large conformational changes of Hsp90 are only weakly coupled to ATP hydrolysis. PMID: 19234469
- Hsp90 os a part of chaperone system that determins molybdate resistance in Saccharomyces cerevisiae. PMID: 19399909
- Data show that the Hsp82 chaperone facilitates telomere DNA maintenance by promoting transitions between two operative complexes and by reducing the potential for binding events that would otherwise block the assembly of downstream structures. PMID: 19525972
- The charged linker region between the N-terminal domain and the middle domain is an important regulator of Hsp90 function. PMID: 19553666
显示更多
收起更多
-
亚细胞定位:Cytoplasm.
-
蛋白家族:Heat shock protein 90 family
-
数据库链接:
KEGG: sce:YPL240C
STRING: 4932.YPL240C
Most popular with customers
-
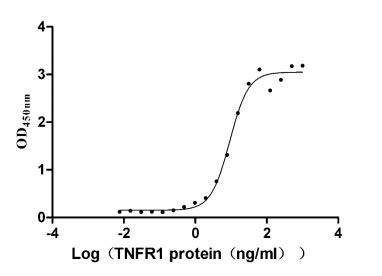
Recombinant Human Tumor necrosis factor receptor superfamily member 1A (TNFRSF1A), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
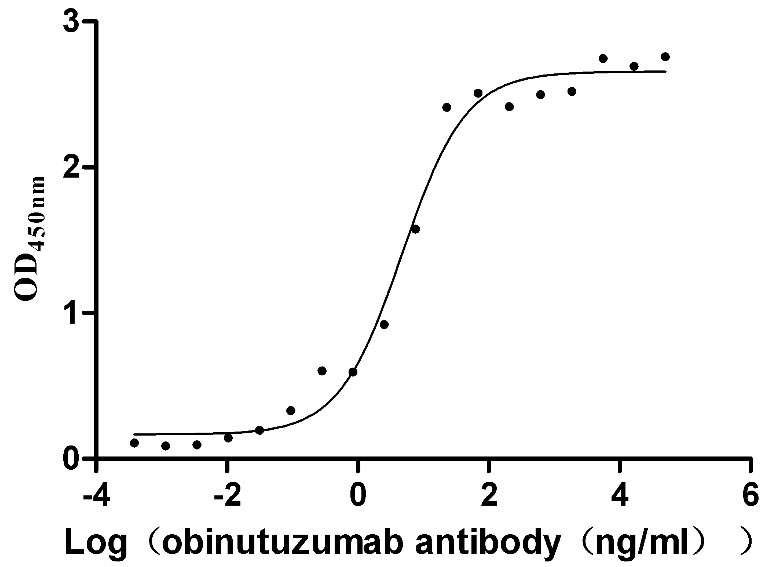
Recombinant Human B-lymphocyte antigen CD20 (MS4A1)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
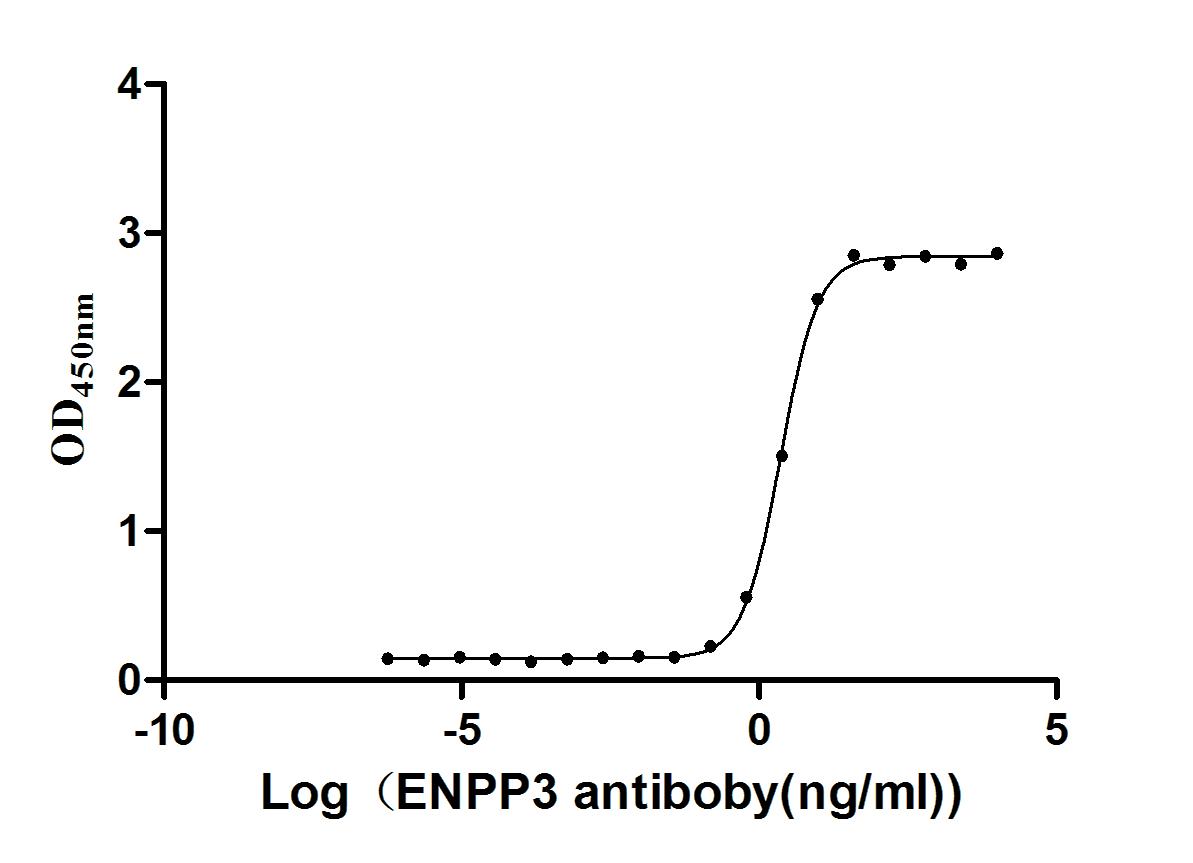
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
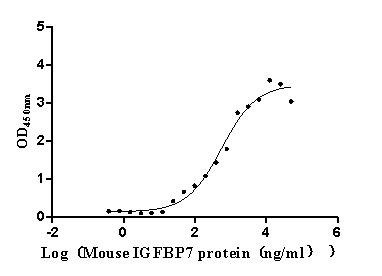
Recombinant Mouse Complement component C1q receptor (Cd93), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
Recombinant Macaca fascicularis Membrane spanning 4-domains A1 (MS4A1)-VLPs (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
Recombinant Human Alkaline phosphatase, germ cell type (ALPG) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Cadherin-17 (CDH17), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)