Recombinant Escherichia coli Multidrug efflux pump subunit AcrB (acrB), partial
In Stock-
中文名称:Recombinant Escherichia coli Multidrug efflux pump subunit AcrB (acrB), partial
-
货号:CSB-EP335613ENV
-
规格:¥2328
-
图片:
-
其他:
产品详情
-
纯度:Greater than 95% as determined by SDS-PAGE.
-
生物活性:Not Test
-
基因名:acrB
-
Uniprot No.:
-
别名:AcrAB-TolC multidrug efflux pump subunit AcrB;Acridine resistance protein B
-
种属:Escherichia coli (strain K12)
-
蛋白长度:Partial
-
来源:E.coli
-
分子量:39.8 kDa
-
表达区域:29-336aa
-
氨基酸序列KLPVAQYPTIAPPAVTISASYPGADAKTVQDTVTQVIEQNMNGIDNLMYMSSNSDSTGTVQITLTFESGTDADIAQVQVQNKLQLAMPLLPQEVQQQGVSVEKSSSSFLMVVGVINTDGTMTQEDISDYVAANMKDAISRTSGVGDVQLFGSQYAMRIWMNPNELNKFQLTPVDVITAIKAQNAQVAAGQLGGTPPVKGQQLNASIIAQTRLTSTEEFGKILLKVNQDGSRVLLRDVAKIELGGENYDIIAEFNGQPASGLGIKLATGANALDTAAAIRAELAKMEPFFPSGLKIVYPYDTTPFVKIS
Note: The complete sequence including tag sequence, target protein sequence and linker sequence could be provided upon request. -
蛋白标签:C-terminal 6xHis-tagged
-
产品提供形式:Liquid or Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
缓冲液:If the delivery form is liquid, the default storage buffer is Tris/PBS-based buffer, 5%-50% glycerol. If the delivery form is lyophilized powder, the buffer before lyophilization is Tris/PBS-based buffer, 6% Trehalose.
-
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:3-7 business days
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4℃ for up to one week.
-
Datasheet & COA:Please contact us to get it.
相关产品
靶点详情
-
功能:AcrA-AcrB-AcrZ-TolC is a drug efflux protein complex with broad substrate specificity that uses the proton motive force to export substrates.; (Microbial infection) Involved in contact-dependent growth inhibition (CDI), acts downstream of BamA, the receptor for CDI. Its role in CDI is independent of the AcrA-AcrB-TolC efflux pump complex.
-
基因功能参考文献:
- It has been demonstrated that an intact AcrAB-TolC efflux pump is crucial to the development of bacterial quinolone resistance. Its activity is complemented by expression of the alternative AcrEF efflux pump. PMID: 28520511
- Data show that the conformational changes occurring in multidrug resistance-associated protein AcrB enable the formation of a layer of structured waters on the internal surface of the transport channel. PMID: 29339082
- the gate loop mutations effects on AcrB solution energetics (fold, stability, molecular dynamics) and on the in vivo efflux of Erythromycin and Doxorubicin. Finally, we discuss the efflux and the discrepancy between the structural and the functional experiments for Erythromycin in these gate loop mutants. PMID: 29126836
- The T37W/A100W double mutant AcrB exports ethidium bromide (EtBr) more actively than wild-type AcrB. PMID: 29317622
- Energy-coupling mechanism of the multidrug resistance transporter AcrB: Evidence for membrane potential-driving hypothesis through mutagenic analysis. PMID: 28497315
- Ten phosphorus ylides were compared in multidrug efflux pump system consisting of the subunits acridine-resistance proteins A and B (AcrA and AcrB) and the multidrug efflux pump outer membrane factor TolC (TolC) and in AcrAB-TolC-deletion. PMID: 27815466
- Data show that CsrA binds directly to the 5' end of the transcript encoding AcrAB. PMID: 29040729
- It has been found that both AcrA and AcrB lasted for approximately 6 days in live E. coli cells, and the stability of AcrB depended on the presence of AcrA but not on active antibiotic efflux. PMID: 27549795
- Two phenylalanine residues located adjacent to the substitution sensitive glycine residues of acrB play a role in blocking the pathway upon rigidification of the loop, since the removal of the phenyl rings from the rigid loop restores drug transport activity PMID: 28987732
- Our data show for the first time effects of various substrate-binding pocket mutations on the kinetics of efflux of two substrates by the AcrB pump. They also confirm interactions between substrates and drugs predicted by MD simulation studies, and also reveal areas that need future research. PMID: 27789287
- Dihydroartemisinine 27 (DHA27) reduced multidrug resistance-associated protein acrB's mRNA expression in a dose-dependent manner. PMID: 27869748
- AcrBA-TolC complex becomes unstable upon the induction of AcrD, which presumably replaces AcrB in the ternary complex. Such instability is suppressed upon the addition of AcrB-specific substrates. The assembly of resistance-nodulation-cell division-type efflux system is a regulated dynamic process that provides bacteria with a highly flexible repertoire of survival strategies to cope with a wide spectrum of antibiotics. PMID: 26916090
- TolC-AcrB interaction only stable on the simulated time scale when both proteins engage in a tip to tip manner. PMID: 27045078
- multiple deletion of acrB, acrD, and mdtABC resulted in a significant decrease in enterobactin export and that plasmids carrying the acrAB, acrD, or mdtABC genes restored the decrease in enterobactin export exhibited by the DeltaacrB acrD mdtABC mutant. PMID: 25259870
- Switch-loop flexibility affects transport of large drugs by the promiscuous AcrB multidrug efflux transporter. PMID: 24914123
- Results suggested that the unfolding of the trimeric AcrB started with a local structural rearrangement. PMID: 24715637
- AcrAB-TolC pump effluxes regulate cellular metabolites that are toxic and have a signaling role. PMID: 24043404
- Genetic defects in SoxS, MarA, or AcrB underlie superoxide-mediated protection of Escherichia coli from antimicrobials. PMID: 23979754
- In addition to the missense mutations in these strains, we detected 7, 20, and 15 nonsense mutations in acrA, acrB, and tolC, respectively. PMID: 24065639
- Data indicate that not all conserved residues are important for AcrB function and not all critical residues are conserved. PMID: 22877148
- analysis of porter domain opening and closing motions in the multi-drug efflux transporter AcrB PMID: 23088914
- used molecular dynamics simulation to examine the binding of nine substrates, two inhibitors, and two nonsubstrates to the distal binding pocket of AcrB, identified earlier by X-ray crystallography PMID: 23175790
- Data show that AcrZ (formerly named YbhT) associates with the AcrAB-TolC complex. PMID: 23010927
- insight from mutagenesis studies on AcrB trimer stability and efflux activity PMID: 22163011
- Four amino acid residues in the vestibule of AcrB play roles role in substrate transport. PMID: 21856849
- The structural characterization results suggest that the oligomerization of AcrB occurs through a three-stage pathway involving folded monomers. PMID: 21664361
- fluoroquinolone resistance appeared to be a stepwise phenomenon, with MIC increasing as point mutations in gyrA increased, but high-level- and multidrug resistance associated with fluoroquinolone resistance reflected overexpression of AcrAB efflux pump PMID: 21194332
- Substrate enters AcrB from the lower part of the cleft before binding to the binding pocket PMID: 20804453
- Performed extensive targeted molecular dynamics simulations mimicking the functional rotation of AcrB containing doxorubicin. PMID: 20548943
- ligands bind not only to the wall of central cavity but also to a new periplasmic site within the deep external depression formed by the C-terminal periplasmic loop PMID: 16166543
- The observed pulsating behavior in inducible transcription of acrB is a systems property arising from the dependence of gene expression on multidrug resistance determinants. PMID: 16461398
- crystal structures of AcrB with and without substrates PMID: 16915237
- crystallographic structure of trimeric AcrB determined at 2.9 and 3.0 angstrom resolution; structural data imply an alternating access mechanism and a novel peristaltic mode of drug transport by this type of transporter PMID: 16946072
- Thr978 functions through hydrogen bonding with Asp407 and protonation of the latter alters the salt bridging and hydrogen bonding pattern in the proton relay network, thus initiating a series of conformational changes that result in drug extrusion. PMID: 17015667
- x-ray structures of four AcrB mutants, the D407A, D408A, K940A, and T978A mutants, in which the structure of this tight electrostatic network is expected to become disrupted; these mutant proteins revealed remarkably similar conformations PMID: 17015668
- The crystal structure of trimeric AcrB in complex with a designed ankyrin-repeat protein inhibitor suggests a transport pathway not through the central pore but through the identified channels in the individual AcrB subunits. PMID: 17194213
- analysis of the TolC/AcrAB complex from E. coli PMID: 17360572
- AcrB forms a mixture of quaternary structures (from monomer to heavy oligomer) in detergent solution. PMID: 17467658
- This study showed that conformational changes, including the closure of the external cleft in the periplasmic domain, are required for drug transport by AcrB. PMID: 17905989
- Measurement of the affinity of ligands to AcrB protein. PMID: 17910961
- The results suggest that AcrA, AcrB and TolC components of multidrug efflux pumps do not associate in an "all-or-nothing" fashion but accommodate a certain degree of flexibility. PMID: 18024521
- The results support the presence of the asymmetric AcrB trimer in E. coli membranes and the functional rotation mechanism. PMID: 18223659
- AcrS represses the gene expression of multidrug efflux genes, acrA and acrB. PMID: 18567659
- Phenylalanine point mutations in the hydrophobic binding pocket in AcrB alters drug susceptibility. PMID: 18849422
- structure of AcrB in complex with deoxycholate at 3.85 A resolution; evidence suggests that bile acid is transported out of the cell via the periplasmic vestibule of the AcrAB-TolC complex PMID: 19023693
- intact cells of E coli containing the intact multiprotein complex AcrB-AcrA-TolC are used to measure the kinetic constants for various cephalosporins PMID: 19307562
- Study conclude that the C-terminal domain of AcrA plays an important role in the assembly and function of AcrAB-TolC efflux pump. PMID: 19411330
- Membrane-located carboxylates play a substantial role as a central element of the proton translocation pathway in AcrB and other members of the resistance nodulation and cell division superfamily. PMID: 19425588
- AcrB trimer shows different conformations of each monomer showing a potential rotational peristaltic pump mechanism for transport PMID: 16946072
显示更多
收起更多
-
亚细胞定位:Cell inner membrane; Multi-pass membrane protein.
-
蛋白家族:Resistance-nodulation-cell division (RND) (TC 2.A.6) family
-
数据库链接:
KEGG: ecj:JW0451
STRING: 316385.ECDH10B_0418
Most popular with customers
-
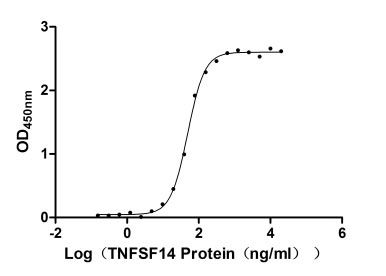
Recombinant Human Tumor necrosis factor ligand superfamily member 14 (TNFSF14), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
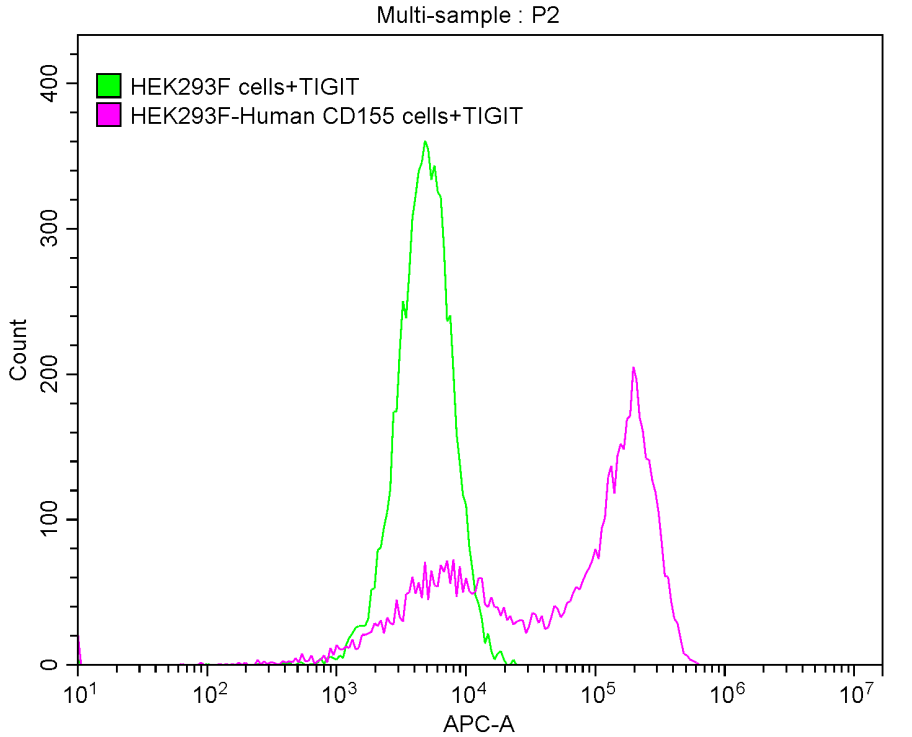
Recombinant Human T-cell immunoreceptor with Ig and ITIM domains (TIGIT), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
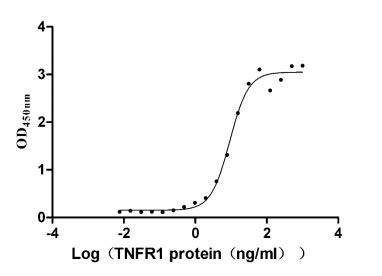
Recombinant Human Tumor necrosis factor receptor superfamily member 1A (TNFRSF1A), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Tumor necrosis factor receptor superfamily member 8 (TNFRSF8), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human C-X-C chemokine receptor type 4 (CXCR4)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human IL12B&IL12A Heterodimer Protein (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Dickkopf-related protein 1 (DKK1) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
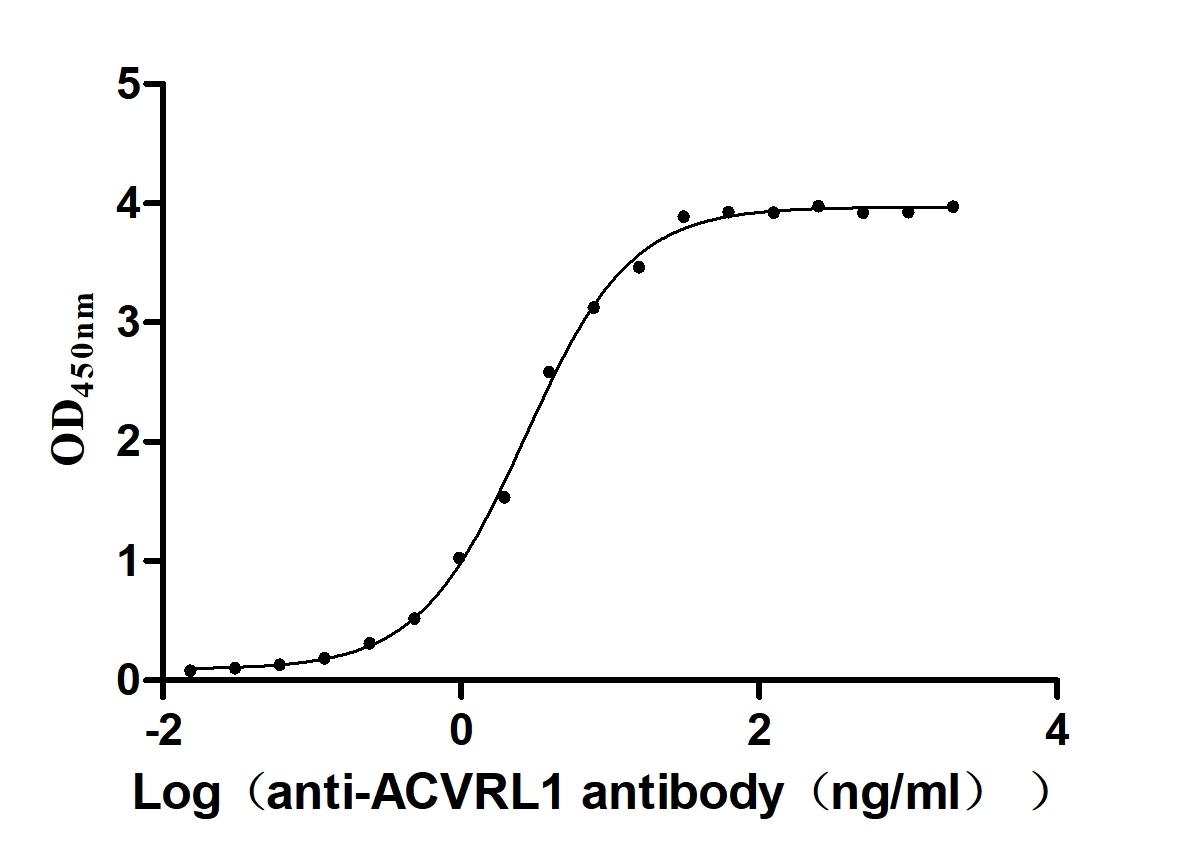
Recombinant Human Serine/threonine-protein kinase receptor R3 (ACVRL1), partial (Active)
Express system: Baculovirus
Species: Homo sapiens (Human)



-AC1.jpg)
-AC1.jpg)