-
中文名称:人血小板生成素受体自身抗体IgG酶联免疫试剂盒
-
货号:CSB-E12990h
-
规格:96T
-
价格:¥3600
-
其他:
产品详情
-
产品描述:本试剂盒(货号CSB-E12990h)采用间接法定性检测人血清样本中抗血小板生成素受体(TPO-R)的自身抗体IgG,适用于科研领域对免疫性血小板减少症相关抗体的基础研究。TPO-R自身抗体可通过干扰血小板生成素信号通路导致血小板减少,与特发性血小板减少性紫癜(ITP)等疾病的免疫病理机制密切相关。本产品采用高特异性包被抗原,通过两步法孵育实现抗体的灵敏捕获,实验流程包含样本稀释、酶标二抗反应及显色分析步骤,显色强度与抗体水平呈正相关。该试剂盒提供预包被板条及完整检测组分,适用于科研场景中探索TPO-R抗体与自身免疫性疾病的关联性、评估免疫调节药物对抗体水平的影响,或作为建立疾病动物模型的辅助检测工具,为免疫性血液疾病的机制研究提供实验支持。实验需自行优化样本稀释比例,具体检测范围建议通过预实验确定。
-
缩写:C-MPL/TPOR auto-Ab (IgG)
-
种属:Homo sapiens (Human)
-
样本类型:serum
-
检测范围:Request Information
-
灵敏度:Request Information
-
反应时间:1-5h
-
样本体积:50-100ul
-
检测波长:450 nm
-
研究领域:Others
-
测定原理:qualitative
-
测定方法:Indirect
-
精密度:
Intra-assay Precision (Precision within an assay): CV%<15%
Three samples of known concentration were tested twenty times on one plate to assess.
Inter-assay Precision (Precision between assays): CV%<15%
Three samples of known concentration were tested in twenty assays to assess.
-
标准曲线:
Test parameter
specification
test result
Positive control
>0.800
1.324
Negative control
<0.1
0.074
Positive rate
10,Positive
100%
Negative rate
10,Negative
100%
-
货期:3-5 working days
引用文献
- Acquired amegakaryocytic thrombocytopenia previously diagnosed as idiopathic thrombocytopenic purpura in a patient with hepatitis C virus infection Shojiro Ichimata.et al,World J Gastroenterol,2017
Most popular with customers
-
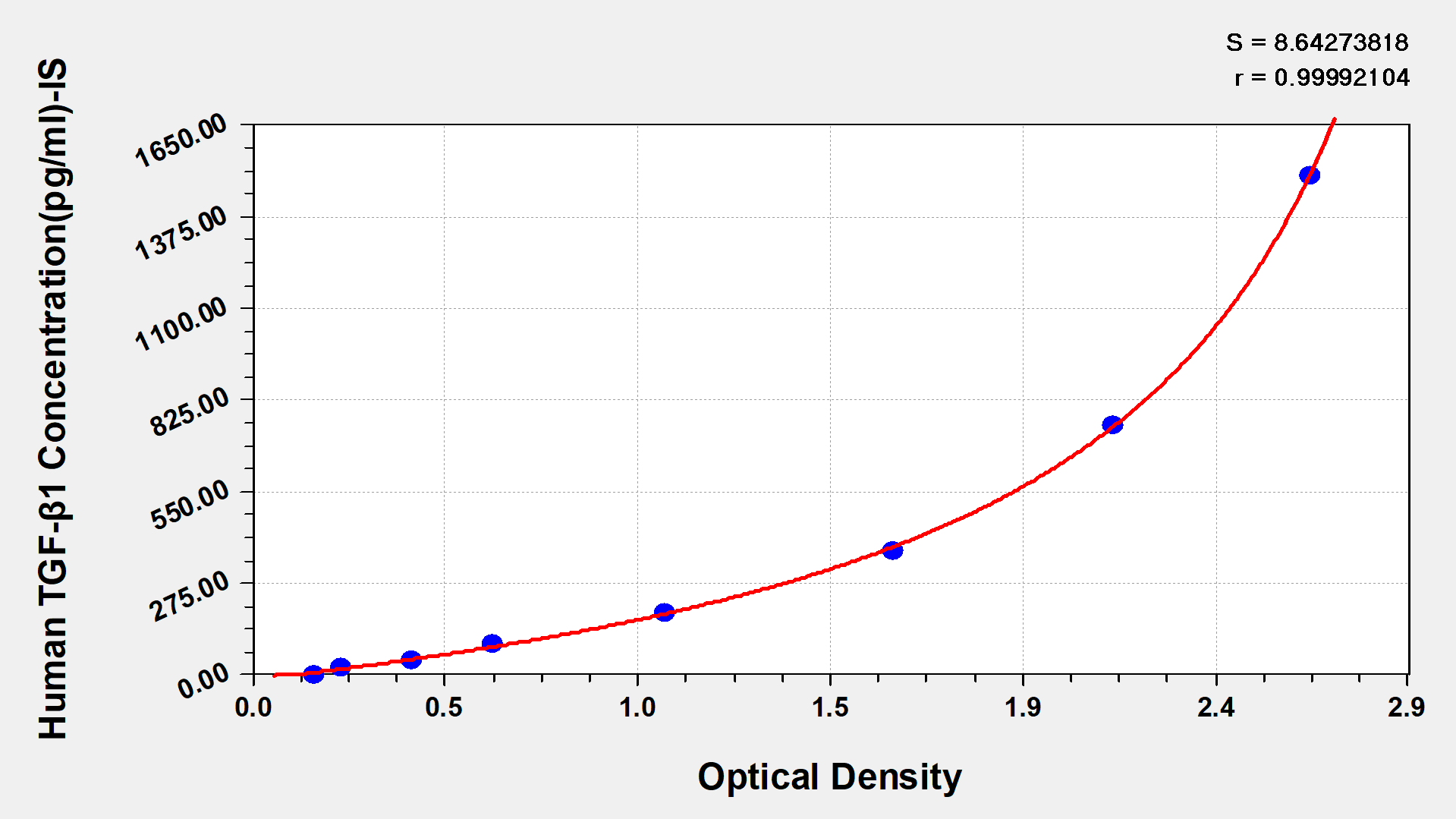
Human Transforming Growth factor β1,TGF-β1 ELISA kit
Detect Range: 23.5 pg/ml-1500 pg/ml
Sensitivity: 5.8 pg/ml
-
-
-
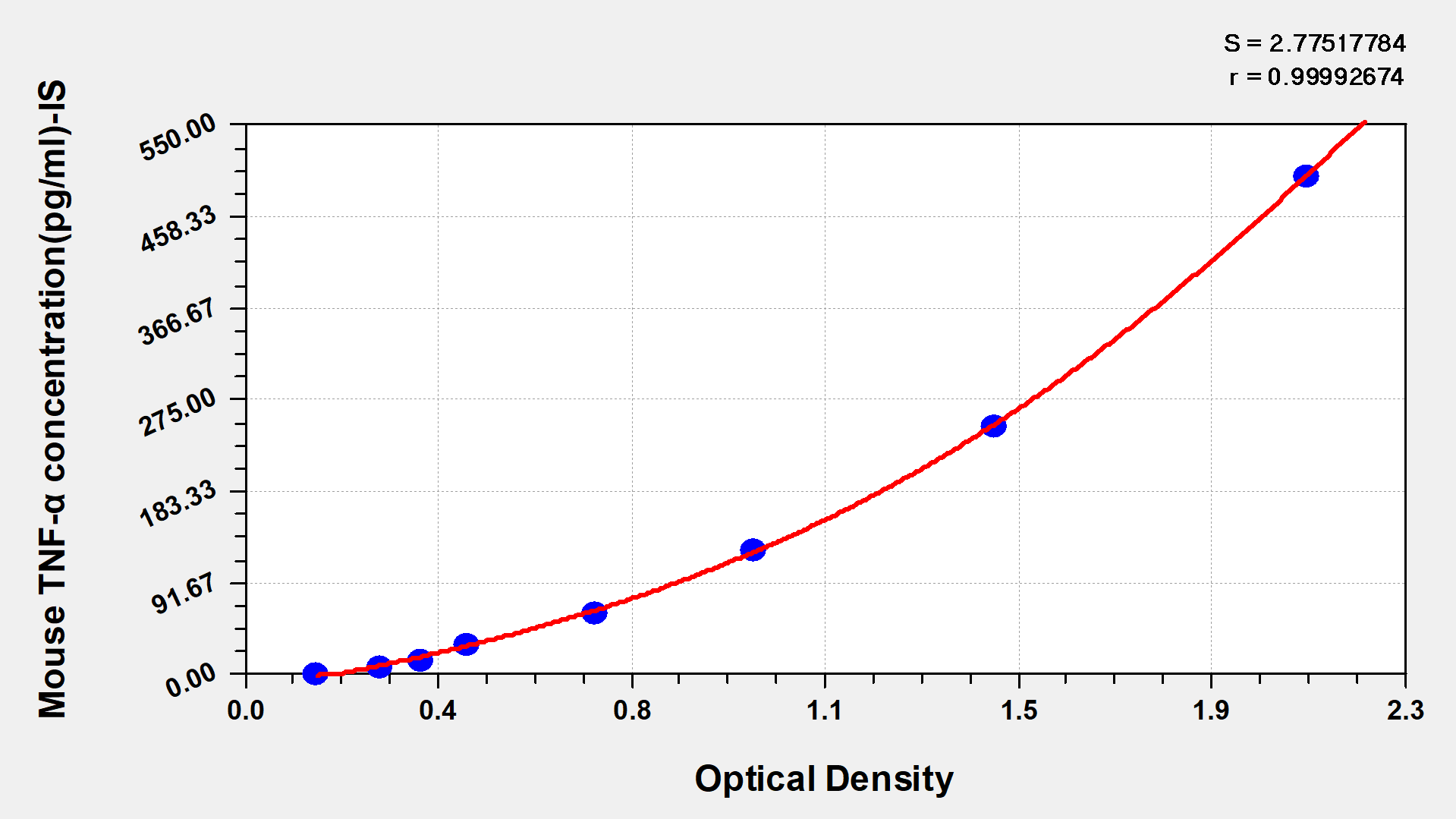
Mouse Tumor necrosis factor α,TNF-α ELISA Kit
Detect Range: 7.8 pg/ml-500 pg/ml
Sensitivity: 1.95 pg/ml
-
-
-
-